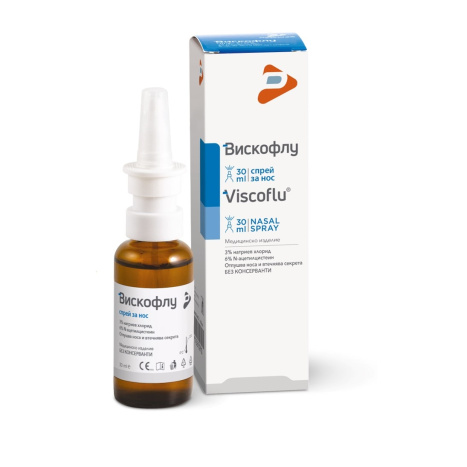
VISCOFLU nasal spray 30ml
3% sodium chloride
6% N-Acetylcysteine
It unclogs the nose and liquefies secretions
30 ml bottle with spray device and protective cap
1. INDICATIONS, COMPOSITION AND PURPOSE OF THE MEDICAL DEVICE
1.1 DESCRIPTION AND INDICATIONS
Viscoflu nasal spray is a medical device composed of 3% hypertonic sodium chloride solution at controlled pH and 6% N-acetylcysteine. It is indicated to facilitate the liquefaction and mechanical removal of mucus and/or mucopurulent secretion located in the nasal cavity (acute, subacute and rhinitis with slow recovery; chronic and mucocrustic rhinitis; vasomotor rhinitis) and in the paranasal sinuses (acute and chronic sinusitis), improving the symptoms and course of acute, subacute and chronic inflammation of the upper respiratory tract. 1.2 CONTRAINDICATIONS AND RESTRICTIONS FOR USE
Hypersensitivity to the active substances or to any of the excipients. Viscoflu nasal spray is contraindicated in children under 2 years of age. Viscoflu nasal spray should be used during pregnancy or breastfeeding only if absolutely necessary and under direct medical supervision.
1.3 COMPOSITION
One 30 ml bottle contains: 1.8 g N-acetylcysteine, 0.9 g sodium chloride. Excipients: sodium hydroxide, disodium edetate, purified water.
1.4 MECHANISM OF ACTION
Viscoflu nasal spray works by reducing the density and viscosity of mucus through the physical/mechanical action of its ingredients:
• pH-controlled hypertonic sodium chloride solution: hypertonic sodium chloride solutions applied directly to the airway mucosa attract water by osmosis. This increases the percentage of water in the mucus overlying the epithelium, thus thinning it and thus facilitating its shedding.
•N-acetylcysteine: the acetylated derivative of the amino acid cysteine demonstrates a mucolytic action in direct contact with mucus. This action is due to the presence of sulfhydryl groups in the molecule, which can destroy the disulfide bridges of the mucoproteins, which are responsible for the viscosity of the mucus, by depolymerizing them.
2. TYPE OF PACKAGING
Solution in a 30 ml glass bottle with attached vertical injection device and protective cap.
3. METHOD OF APPLICATION
For proper use: remove the protective cap, tilt your head back slightly, carefully insert the injector into the nostril opening, press the base of the injector using two fingers and spray 2-3 times in a row. Repeat the procedure in the other nostril. Clean the injector and replace the protective cap. Shake well before use.
3.1 DOSAGE, METHOD AND DURATION OF ADMINISTRATION
The recommended dose is 2-3 sprays in each nostril two to three times daily for no more than 10 days, depending on the clinical symptoms being treated. This treatment can be repeated several times during the year, and the use of the product must be suspended for at least 1 week between individual cycles. To be used within 2 months of opening the bottle.
3.2 KNOWN ADVERSE REACTIONS
The high concentration of salt in the solution may cause a burning sensation after injection, which is normal; this effect disappears within a few minutes. Adherence to the instructions in the leaflet reduces the risk of side effects.
4. CONDITIONS OF STORAGE
Store at room temperature (not higher than 25℃) away from light and heat sources. This expiration date applies to product that has not been opened and has been stored properly.
5. WARNINGS AND PRECAUTIONS
After spraying from the spray, Viscoflu nasal spray has a smell of sulfur, which does not change the product in any way. After each injection, wash the injection device. It is recommended to replace the protective cap after use. Do not use in case of hypersensitivity or allergy to any of the product ingredients.
Prolonged use of topical products may lead to sensitization; if such effects occur, discontinue treatment and consult your physician.
Keep the product out of the reach of children. Do not use the medical device after the expiration date. Do not use the medical device if the packaging is damaged. Do not swallow it. Do not spray in eyes. The use of the same bottle by more than one person can facilitate the transmission of infections.
6. DISPOSAL
When the expiration date has passed or the product has to be discarded, the product must be disposed of in accordance with the local waste handling requirements of the relevant competent authorities. In any case, dispose of the product and its packaging responsibly.
Pharma Line Srl Via Bertani 2,
20154 Milan, Italy
Distributor:
Orbiko Bulgaria EOOD, Chelopeshko Shose St. 24, 1839 Sofia Phone: +359 (2) 40 24 500
Email: info.bg@orbico.com
1.8 g of N-acetylcysteine, 0.9 g of sodium chloride. Excipients: sodium hydroxide, disodium edetate, purified water