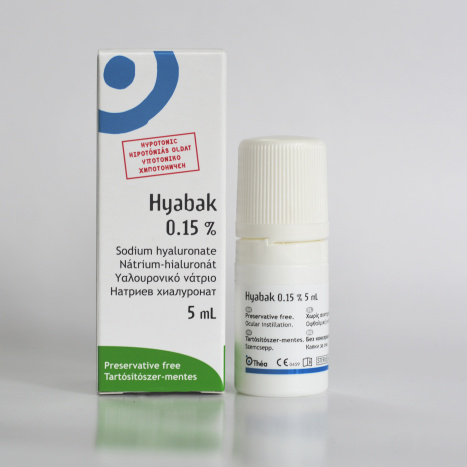
HYABAK for moisturizing eyes and contact lenses 5ml
INSTRUCTIONS FOR USE
HYABAK®
This product is a medical device. Please read the instructions before use.
Keep this leaflet. You may need to read it again.
INDICATIONS
HYABAK is designed to moisturize and lubricate the eye and contact lenses.
For ocular use only.
APPROPRIATE FOR
- For adults only,
- Wearing all types of contact lenses.
CONTRAINDICATIONS
Do not inject or swallow.
Do not use HYABAK if you are allergic to any of its ingredients (see Product Description).
Due to the lack of sufficient data, do not use HYABAK if you are pregnant or breastfeeding, unless recommended by your doctor.
Due to the lack of sufficient data, HYABAK should not be used in children unless recommended by your doctor.
PRODUCT DESCRIPTION
HYABAK is an ophthalmic solution containing sodium hyaluronate (0.15%), sodium chloride, actinoquinol, trometamol, hydrochloric acid and water for injections.
HYABAK is a sterile, phosphate-free, hypotonic and pH neutral solution.
The solution is available in an ABAK® multidose bottle. This innovative and patented device makes it possible to apply the eye drops through a 0.2 µm filter, protecting the solution from bacterial contamination, without the presence of any preservatives.
Therefore, HYABAK is gentle on the eyes as it contains no preservatives. The absence of preservatives allows the protection of eye tissues and also does not cause reactions on the part of users who are sensitive to preservatives.
BENEFIT, PRESENTATION AND MECHANISM OF ACTION
HYABAK is recommended for moisturizing and lubricating the eyes in case of feeling of dryness and fatigue of the eyes caused by external factors, such as wind, tobacco smoke, pollution, dust, dry heat, air conditioning, traveling and prolonged standing in front of a computer screen. It provides immediate eye relief and comfort throughout the day.
HYABAK is also recommended for contact lens wearers. It lubricates and moisturizes the lenses, makes them easier to insert or remove, and to wear them all day.
HYABAK's main ingredient is sodium hyaluronate, a natural polysaccharide found normally in the human eye that holds water to hydrate (wet) and lubricate the surface of the eye. It holds the solution on the surface of the eye, providing long-lasting relief and shortening the healing time of the ocular surface.
HYABAK also contains actinoquinol (a substance with anti-UV properties).
GENERAL APPLICATION RECOMMENDATIONS
No special training is required before applying HYABAK.
Wash your hands thoroughly before use.
Do not use any tools to open the bottle.
When using HYABAK for the first time, you will notice that it takes longer for the first drop to come out due to the unique filtering system in the bottle (see Product Description).
After use, put the cap back on the bottle.
To moisturize and lubricate the eye:
Dosage: 1 drop in each eye, as many times as needed during the day.
Place one drop in the eyelid pocket by gently pulling the lower eyelid down and looking up. Carefully close the eye after inserting the drop.

To lubricate and hydrate the lenses :
Dosage: 1 drop on each lens when inserting and removing it, also as many times as needed during the day.
Before putting your contact lenses in, simply place one drop into the inner recess of the lens when it is on your finger.
Put your contact lenses in as usual and blink a few times to help distribute HYABAK evenly over the eye and contact lens surface.

PRECAUTIONS FOR USE
The product is intended for repeated use by one patient.
Do not pass the bottle on to other people, to prevent so-called cross-contamination.
Check that the packaging is not damaged before using this product for the first time.
Check that the safety ring of the bottle is intact before using it for the first time.
To avoid contamination, do not touch the tip of the bottle to any surface.
You should seek medical attention if your symptoms worsen or do not improve.
WARNINGS
Keep out of the reach of children.
You should not drive or operate machinery if your vision is blurred after use: wait five minutes for your vision to recover (clear).
Wait at least 10 minutes between applying another eye product.
Never dilute HYABAK or mix it with other solutions.
After the first use, if exposed to low atmospheric pressure, the bottle may leak.
A UV filter does not replace wearing sunglasses.
UNWANTED REACTIONS
The following side effects have been reported: rare cases of mild irritation and redness of the eyes.
In these cases, contact lens wearers should remove them.
When using eye drops, you may experience some symptoms of discomfort, such as a burning sensation, "stinging", sensation of a foreign body in the eye and blurred vision for a short period of time.
If you notice any side effects or unusual sensation after using this product, please consult your doctor and report this information to the local distributor, manufacturer or your local health authority (see contact information at the end of the instructions for use).
STORAGE AND DISPOSAL CONDITIONS
Store between 8°C and 30°C.
Do not expose your product to direct sunlight or moisture.
Do not use after the expiry date stated on the bottle and carton.
The expiration date corresponds to the last day of the specified month, in case the packaging has not been opened and stored properly.
The product can be used up to 3 months after first opening. After this period, dispose of the remaining product as required.
Dispose of the product responsibly according to local regulations (the bottle must be disposed of in the household waste container, the carton and leaflet can be recycled).
Date of issue and version of the instruction manual: Ver.3 11/2019
0459

 Manufacturer :
Manufacturer :
Laboratoires Théa
12 rue Louis Blériot
63017 Clermont-Ferrand Cedex 2 France
«Contact Information»
Bulgaria:
Distributor : Tea Pharma OOD, Sofia 1113, Shipchenski Prohod Blvd. 18, Galaxy Shopping Center, Office 110, Bulgaria
Health authority : Executive Agency for Medicines, Sofia 1303, 8 "Damyan Gruev" St., Bulgaria
T1552 |
MEANING OF THE PICTOGRAMS ON THE PACKAGING
| Manufacturer Manufacturer |
| Medical Device Medical product |
| Batch number Lot |
| Consult instructions for use Read the instructions for use |
| Date of manufacture Date of manufacture | Single patient – Multiple use Single Patient-Multiple Use | |
| Use-by date Good until |
| Do not use if package is damaged Do not use if packaging is damaged |
| Temperature limits Temperature conditions of storage |
| Caution Caution |
| Sterilized using aseptic processing techniques Sterilized by aseptic techniques | Catalog number Catalog number |