MEZYM FORTE / Mezym Forte 10000 x 50 tabl
LEAFLET
Patient information leaflet
Mezim â forte 10000 Ph. Eur. U. gastro-resistant tablets
Mezymâ forte 10000 Ph. Eur. U. gastro-resistant tablets
Pancreatine ( Pancreatine )
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you.
- Keep this leaflet.
- If you need further information or advice, ask your pharmacist.
- If you get any side effects, please tell your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- If you do not feel better after 7-14 days or your condition worsens, you should seek medical attention.
What this leaflet contains:
1. What Mezim ® forte 10000 Ph. Eur. U. is and what it is used for
2. What you need to know before you take Mezim ® forte 10000 Ph. Eur. U.
3. How to take Mezim ® forte 10000 Ph. Eur. U.
4. Possible side effects
5. How to store Mezim ® forte 10000 Ph. Eur. U.
6. Contents of the pack and other information
- What is Mezim ® forte 10000 Ph. Eur. U. and what is it used for?
What is Mezim ® forte 10000 Ph. Eur. U. ?
Mezim ® forte 10000 Ph. Eur. U. is a medicine containing substances (enzymes) from pig pancreas (pancreatic powder, also called pancreatin) that aid digestion.
What is Mezim ® forte 10000 Ph. Eur. U. used for ?
Mezim ® forte 10000 Ph. Eur. U. is used to replace digestive enzymes in case of indigestion resulting from reduced or absent pancreatic function (exocrine pancreatic insufficiency).
- What you need to know before taking Mezim ® forte 10000 Ph. Eur. U.
Do not take Mezim ® forte 10000 Ph. Eur. U .:
- if you are allergic to pancreatin, pork products, azorubine (E 122) or any of the other ingredients of this medicine (listed in section 6);
-if you suffer from acute inflammation of the pancreas or exacerbated chronic inflammation of the pancreas during the active phase of the disease. However, it is sometimes advisable to use the medicine in the recovery phase with a diet (light food) if the indigestion persists.
Warnings and precautions
Talk to your doctor or pharmacist before taking Mezim ® forte 10000 Ph.
Eur. U.:
-if you notice that you have complaints similar to intestinal obstruction (e.g. abdominal pain, lack of bowel function, nausea, vomiting).
Intestinal obstruction is a known complication in patients with cystic fibrosis;
-If you experience unusual stomach or gastrointestinal discomfort or changes in symptoms, as a precaution you should be examined by your doctor to rule out the possibility of intestinal damage. This mainly affects patients taking more than 10,000 units of lipase per kilogram of body weight per day.
-this medicine contains active enzymes which, when released into the oral cavity, e.g. when chewing the tablet, may cause damage to the oral mucosa (e.g. oral ulcers). Therefore, care should be taken to ensure that Mezim® forte 10000 Ph. Eur. U. tablets are taken whole.
Other medicines and Mezim ® forte 10000 Ph. Eur. U.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Folic acid absorption (the passage of folic acid into the blood) may be reduced by taking medicinal products containing pancreatin, which means that additional folic acid intake may be required.
The effect of oral blood sugar lowering drugs (oral antidiabetic drugs) acarbose and miglitol may be reduced by simultaneous administration of Mezim ® forte 10000 Ph. Eur. U.
Pregnancy and breastfeeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to become pregnant, ask your doctor or pharmacist for advice before using this medicine.
There is insufficient data on the use of Mezim ® forte 10000 Ph. Eur. U. in pregnant women. There are only insufficient data from animal studies on pregnancy, development of the unborn child, birth and development of the child after birth. The possible risk in humans is unknown. If you are pregnant or breastfeeding, you should not take Mezim ® forte 10000 Ph. Eur. U. unless your doctor considers that the intake is absolutely necessary.
Driving and using machines
Mezim ® forte 10000 Ph. Eur. U. has no or negligible effect on the ability to drive and use machines.
Mezim ® forte 10000 Ph. Eur. U. contains lactose and azorubine
If your doctor has told you that you have an intolerance to some sugars, contact your doctor before taking this medicine.
This medicinal product contains the colouring agent azorubine (E 122), which may cause allergic reactions.
- How to take Mezim ® forte 10000 Ph. Eur. U.
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Dosage
The recommended dose is:
2-4 tablets Mezim ® forte 10000 Ph. Eur. U. during meals (equivalent to 20,000-40,000 units of lipase per meal).
The dosage of Mezim ® forte 10000 Ph. Eur. U. is determined by the severity of the existing digestive disorder. The required dose may be even higher. A greater increase in the dose can only be carried out under medical supervision and is aimed at improving symptoms (e.g. fatty bulky stools, abdominal pain).
A daily enzyme dose of 15,000 – 20,000 lipase units per kilogram of body weight should not be exceeded. Especially in patients with cystic fibrosis, the enzyme dose should not be higher than that required for sufficient fat absorption.
Use in children
The dosage for children should be determined by a doctor.
Method of administration
Mezim ® forte 10000 Ph. Eur. U. tablets should be swallowed whole with plenty of liquid (water or juice) during meals. Please note that the tablets should be swallowed whole, as chewing reduces the effectiveness of Mezim ® forte 10000 Ph. Eur. U. and the enzymes contained in them may, after release, damage the oral mucosa. Then take plenty of liquid (water or juice).
Duration of treatment
The duration of treatment is unlimited. The duration of use of Mezim ® forte 10000 Ph. Eur. U. corresponds to the duration of the disease and is determined by a doctor. Please talk to your doctor if you do not feel better or if you feel worse.
Please consult your doctor or pharmacist if you have the feeling that the effect of Mezim ® forte 10000 Ph. Eur. U. is too strong or too weak.
If you take more Mezim ® forte 10000 Ph. Eur. U than you should
Then drink plenty of water and talk to your doctor or pharmacist.
Extremely high doses of pancreatin, especially in patients with cystic fibrosis, may lead to an increase in uric acid in the blood (hyperuricemia) and urine (hyperuricosuria).
If you forget to take Mezim ® forte 10000 Ph. Eur. U.
Do not take a double dose to make up for a forgotten tablet and continue treatment as recommended.
If you stop taking Mezim ® forte 10000 Ph. Eur. U.
If you stop treatment with Mezim ® forte 10000 Ph. Eur. U. early or if you interrupt treatment, the symptoms of indigestion may reappear. Talk to your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
- Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Important side effects or signs to look out for and appropriate measures to take
If you are affected by any of the listed side effects, do not take Mezim ® forte 10000 Ph. Eur. U. any more and, if possible, consult a doctor immediately. He will assess further treatment.
Very rare side effects (may affect less than 1 in 10,000 patients)
- diarrhea, abdominal discomfort, abdominal pain, nausea, vomiting;
- immediate allergic reactions, such as skin rash, hives, sneezing, tearing, shortness of breath due to narrowing of the airways (bronchospasm), difficulty breathing;
- allergic reactions of the digestive system;
- Narrowing of the small intestine/appendiceal area and descending colon has been described in patients with cystic fibrosis when given high doses of pancreatin. This narrowing may lead to intestinal obstruction (see section 2 “Warnings and precautions”).
Side effects with unknown frequency (frequency cannot be estimated from the available data)
- In patients with cystic fibrosis, especially when taking high doses of pancreatin, increased urinary excretion of uric acid may be observed. Therefore, if you are a patient with cystic fibrosis, your doctor should monitor the urinary excretion of uric acid to avoid the formation of urate stones.
- Azorubine (E 122) may cause allergic reactions.
Reporting side effects
If you get any side effects, please tell your doctor or pharmacist. This includes any side effects not listed in this leaflet. You can also report side effects directly via the national reporting system:
Executive Agency for Medicines
8 Damyan Gruev Street
1303 Sofia
tel.: +359 28903417
website: www.bda.bg
By reporting side effects, you can help provide more information about the safety of this medicine.
- How to store Mezim ® forte 10000 Ph. Eur. U.
Keep out of the reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after “EXP”. The expiry date refers to the last day of that month.
Storage conditions
Store below 30 °C!
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to dispose of medicines you no longer use. This will help to protect the environment.
- Package contents and additional information
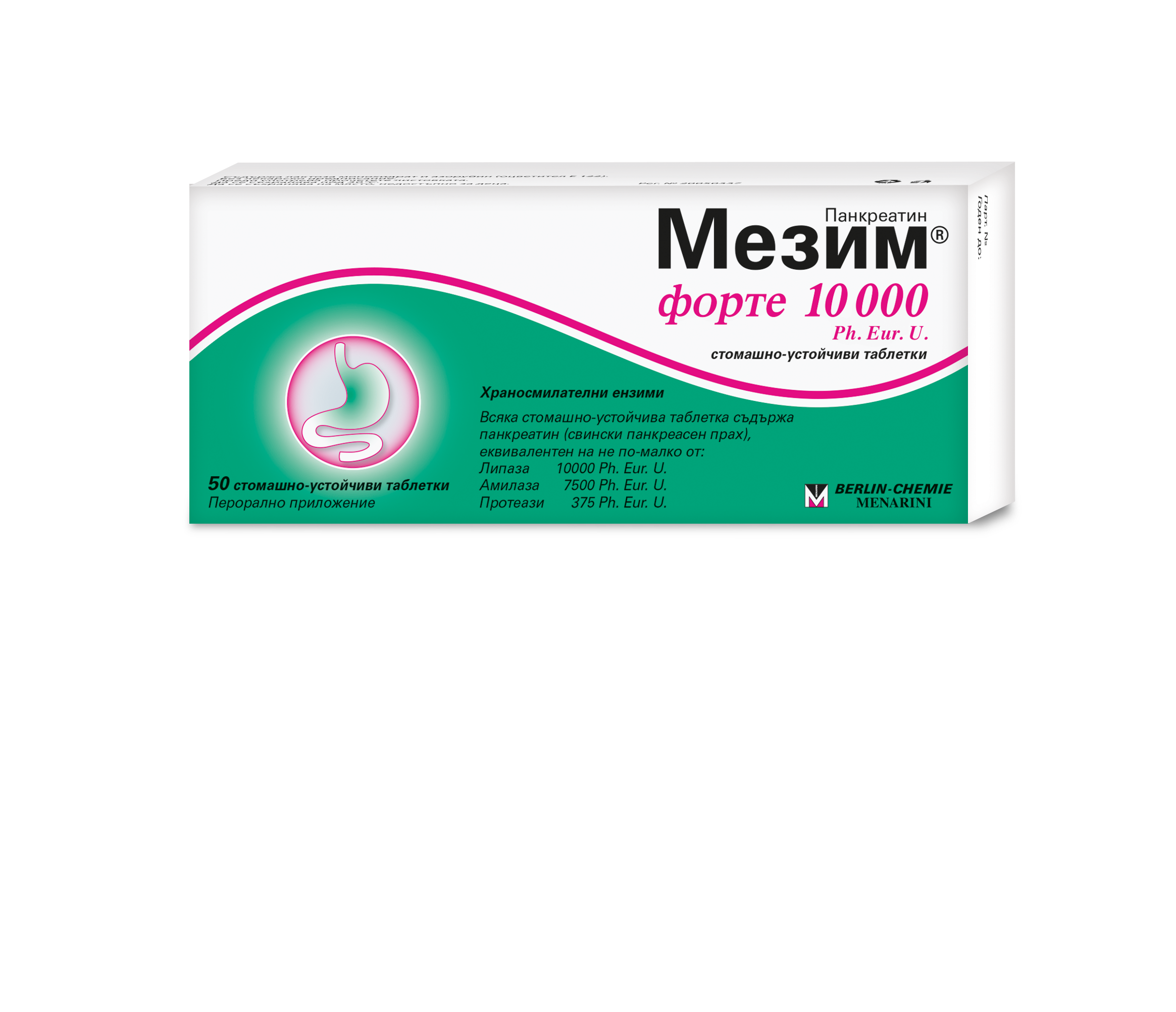
What does Mezim ® forte 10000 Ph. Eur. U contain?
The active substance is:
One gastro-resistant tablet contains 80 mg – 111,111 mg pancreatin (porcine pancreas powder), equivalent to not less than the following activity:
Lipase 10000 Ph. Eur. Units/tablet
Amylase not less than 7500 Ph. Eur. Units/tablet
Proteases not less than 375 Ph. Eur. Units/tablet
Pancreas powder is produced from pig pancreatic tissue.
The other ingredients are:
Core: lactose monohydrate, microcrystalline cellulose, crospovidone, colloidal anhydrous silica, magnesium stearate (Ph. Eur.) (vegetable).
Coating: hypromellose, methacrylic acid - ethyl acrylate copolymer (1:1 dispersion) 30%, triethyl citrate, talc, simethicone emulsion consisting of simethicone, methylcellulose, sorbic acid (Ph. Eur), water; macrogol 6000, carmellose sodium, polysorbate 80 (vegetable), azorubine (colorants E 122), titanium dioxide, sodium hydroxide.
What Mezim ® forte 10000 Ph. Eur. U. looks like and what the pack contains
Mezim ® forte 10000 Ph. Eur. U. are pink, round, slightly rounded tablets with beveled edges with gastro-resistant film coating. They are available in blisters in cardboard packaging, together with a patient leaflet.
Original packages with 10, 20, 50 or 100 tablets.
Not all types of packaging can be released for sale.
Marketing Authorisation Holder and Manufacturer
Berlin-Chemie AG (MENARINI GROUP)
Glienicker Weg 125
12489 Berlin
Germany
Date the leaflet was last revised
July, 2018