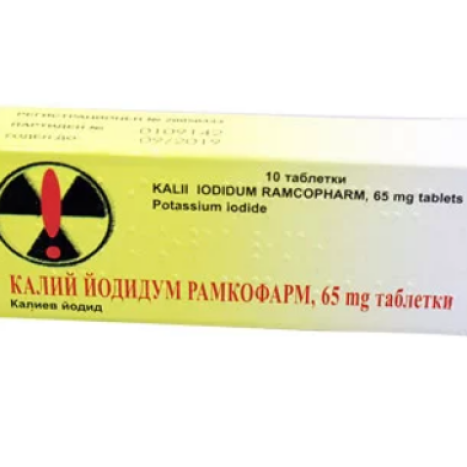
KALII IODIDUM Ramcopharm 65mg x 10 tabl
Iodine tablets
LEAFLET: INFORMATION FOR THE USER
KALI Y IODIDUM RAMKOPHARM 65 mg tablets
K ALII IODIDUM RAMCO PHARM 65 mg tablets
Potassium iodide _ _
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you.
- Keep this leaflet. You may need to read it again.
- If you need further information or advice, ask your pharmacist.
- If you get any side effects, tell your doctor or pharmacist. This also includes any possible side effects not described in this leaflet. See point 4.
- If after 2-3 days you do not feel well, stop this medicine.
What this leaflet contains
1. What is KALI Y IODIDUM RAMKOPHARM 65 mg tablets
and what it is used for.
2. What you need to know before you take KALI Y IODIDUM RAMKOPHARM 65 mg tablets
3. How to take KALI Y IODIDUM RAMKOPHARM 65 mg tablets
4. Possible side effects.
5. How to store POTASSIUM IODIDUM RAMKOPHARM 65 mg tablets .
6. Contents of the package and additional information.
WHAT IS KALI Y IODIDUM RAMKOPHARM 65 mg tablets
AND WHAT IT IS USED FOR Taking potassium iodide immediately before or up to 3 hours after an incident involving the release of radioactive isotopes of iodine and/or technetium blocks the accumulation of these substances in the thyroid gland and thus protects it from the damaging effects of ionizing radiation, which they emit. Prophylaxis with potassium iodide reduces the risk of suppression of thyroid gland functions and development of thyroid cancer when the body is exposed to iodine or technetium radionuclides.
l WHAT IT IS USED FOR
To block or reduce the accumulation of radioactive isotopes of iodine in the thyroid gland and respectively reduce the risk of hypothyroidism (suppression of thyroid gland functions) and thyroid cancer in the conditions of incidents related to radioactive contamination and release of iodine and technetium radioactive isotopes (accidents in nuclear power plants, terrorist acts, etc.).
2. BEFORE YOU TAKE KALI Y IODIDUM RAMKOPHARM 65 mg tablets
l When you should not take KALI Y IODIDUM RAMKOPHARM 65 mg tablets
POTASSIUM IODIUM RAMCOPHARM 65 mg tablets SHOULD NOT BE TAKEN IF
- You suffer from a thyroid disease or have had a similar disease in the past
- In case of hypersensitivity to iodine or any of the excipients
- You suffer from dermatitis herpetiformis
- You suffer from hypocomplementary vasculitis (a type of inflammation of the blood vessels)
Please, if you have any questions or doubts, contact your doctor or pharmacist!
l WARNINGS AND PRECAUTIONS
Talk to your doctor or pharmacist before taking KALI Y IODIDUM RAMKOPHARM 65 mg tablets
To ensure maximum effectiveness of POTASSIUM IODIDUM RAMKOPHARM 65 mg tablets, it is necessary to take it immediately after exposure or immediately before. Note that potassium iodide does not provide protection against the immediate effects induced by ionizing radiation and does not suppress the absorption of ionizing radiation by the body.
Prophylaxis with the medicinal product does not cancel the need for immediate evacuation, especially of infants, small children, pregnant and lactating women from the areas located near the accident, nor does it exclude the need to provide shelter and deliver uncontaminated food, water, etc. A single dose of potassium iodide provides effective protection against the "accumulation" of radioactive iodine isotopes for about 24 hours, but when evacuation and/or the provision of uncontaminated food is impossible, daily intake of the medicinal product within the exposure is required, the intake period being desirable of KALI Y IODIDUM RAMKOPHARM 65 mg tablets should not exceed 10 days.
In infants under the age of 1 month, it is recommended to administer a single dose of POTASSIUM IODIDE RAMKOFARM 65 mg tablets , providing protection of the thyroid gland from the accumulation of radioactive iodine isotopes for 24 h and at the same time ensuring uncontaminated food products and immediate evacuation. It is desirable to avoid prolonged intake of the medicinal product in this group of patients. When using potassium iodide prophylaxis, infants under 1 month of age should be carefully monitored for hypothyroidism (reduced thyroid function).
In children and adolescents (from 1 month to 18 years), there is a high risk of damage to the thyroid gland from radioactive isotopes of iodine, but the risk of adverse effects of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets is relatively low. For this reason, in cases where the risk of inhalation or intake of contaminated milk or food is longer, it is necessary to take the recommended dose daily. However, it is desirable that the duration of reception does not exceed 10 days.
In elderly patients, pregnant women and nursing mothers, it is desirable to avoid taking more than one dose of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets , except in exceptional cases where there is prolonged contact with radioactive isotopes (through inhalation or intake with contaminated food). . Ingestion of potassium iodide by pregnant and lactating women may cause rashes or hypothyroidism (suppression of thyroid function) in the newborn/infant. It is recommended that during the period during which a nursing woman takes potassium iodide, breastfeeding should be temporarily stopped (usually 1-2 days) until evacuation to a safe area.
In individuals over the age of 40, the risk of thyroid cancer after exposure to radioactive isotopes is extremely low, while the risk of adverse reactions to potassium iodide increases with age. In these patients, prophylaxis with potassium iodide is indicated only for high doses of ionizing radiation, which can realistically be expected only in patients located in areas close to the incident.
The risk of side effects of POTASSIUM IODIUM RAMKOPHARM 65 mg tablets (especially hyperkalemia) increases in people with kidney diseases, and therefore the condition of such patients should be carefully monitored.
The medicinal product should be used with caution in patients with congenital myotonia (muscle weakness) and hyperkalemia (increased potassium levels in the blood) with careful monitoring of potassium levels.
In patients with tuberculosis , POTASSIUM IODIDE RAMKOPHARM 65 mg tablets should be used with caution due to the risk of significantly increasing the release of bronchial secretions.
l OTHER MEDICINES AND POTASSIUM IODIDE RAMKOPHARM 65 mg tablets
You must tell your doctor or pharmacist if you are taking, have recently taken, or are likely to take other medicines (prescription or non-prescription) together with KALI Y IODIDUM RAMKOPHARM 65 mg tablets .
The simultaneous use of potassium-sparing diuretics, ACE inhibitors, as well as other potassium-containing medicinal products or nutritional supplements increases the risk of hyperkalemia (increased plasma potassium concentrations) and related effects on cardiac function. Taking POTASSIUM IODIDE RAMKOPHARM 65 mg tablets together with lithium salts or thyrostatics (medicines that suppress the function of the thyroid gland) can increase the effects of these medicines. The simultaneous use of KALIJ IODIDUM RAMKOPHARM 65 mg tablets and drugs containing iodine, such as amiodarone, clioquinol, contrast diagnostic agents and iodine-containing antiseptics (especially when treating infants and extensive body surfaces) increases the risk of damage to the function of the thyroid gland.
l PREGNANCY, LACTATION AND FERTILITY
If you are pregnant or breast-feeding, think you may be pregnant or are planning to become pregnant, ask your doctor or pharmacist for advice before using this medicine.
Administration of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets during pregnancy is imperative, given the risk of accumulation of radioactive isotopes of iodine in the thyroid gland of the fetus. It is recommended that pregnant and lactating women receive 2 tablets of 65 mg as a single dose and be immediately removed from the site of exposure. POTASSIUM IODIDE RAMKOPHARM 65 mg tablets should be taken again only in case of unavoidable prolonged exposure. It is advisable to closely monitor neonates for signs of thyroid suppression and/or goiter.
Due to the risk of overdose and for the purpose of more accurate dosing for the infant, it is recommended to stop breastfeeding (for 1-2 days) when the lactating woman takes potassium iodide. Iodine is excreted in breast milk, and up to 25% of the amount received by the mother can be eliminated in breast milk in 24 hours. It is desirable for lactating women to apply only a single dose of the medicinal product (except in cases of prolonged exposure). In cases where it is necessary to take more than one tablet of the medicinal product, it is desirable to carefully monitor infants for signs of iodism, suppression of thyroid function or rashes.
l DRIVING AND OPERATING MACHINERY
POTASSIUM IODIUM RAMKOPHARM 65 mg tablets does not cause drowsiness and dizziness and can be used by drivers and machine operators.
l IMPORTANT INFORMATION ABOUT SOME OF THE INGREDIENTS OF POTASSIUM IODIDE RAMKOPHARM 65 mg tablets
This medicine does not contain excipients that can affect the human body.
3. HOW TO TAKE POTASSIUM IODIUM RAMCOPHARM 65 mg tablets
l METHOD AND ROUTE OF ADMINISTRATION
POTASSIUM IODIUM RAMKOPHARM 65 mg tablets are taken orally with a glass of water.
l THE RECOMMENDED DOSAGE IS:
Adults and children over 12 years old – 2 tablets of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets once a day (equivalent to 100 mg of iodine);
Pregnant and lactating women : 2 tablets of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets (equivalent to 100 mg of iodine). It is recommended that pregnant and lactating women take POTASSIUM IODIUM RAMKOPHARM 65 mg tablets only once and immediately be taken to a safe, uncontaminated place.
- Children from 3 to 12 years: 1 tablet of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets (equivalent to 50 mg of iodine). Adolescents with a body weight of 70 kg or more should take the adult dose.
Children aged 1 month to 3 years : 1/2 tablet of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets (equivalent to 25 mg iodine)
Infants (up to 1 month old): 1/4 tablet of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets (equivalent to 12.5 mg iodine)
The indicated recommended doses should be taken as soon as possible after the accident and suspected exposure (within 3 hours after the accident). It is necessary to take one dose every 24 hours as long as there is a risk of inhalation or intake with food and liquids of radioactive isotopes. In newborns (under 1 month), pregnant women and nursing mothers, it is recommended to take only a single dose (See Special warnings and precautions for use).
The tablets should be taken after a meal and can be taken whole with plenty of liquid or crushed and mixed with drinks or milk.
Always take this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you. If you are not sure about something, ask your doctor or pharmacist.
l If you have taken more than the required dose of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets.
Do not exceed the indicated dose. In case you accidentally take too many tablets of POTASSIUM IODIDE RAMKOPHARM 65 mg tablets , immediately contact your doctor for help.
If you have any further questions related to the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE ADVERSE REACTIONS
As with all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any of the following unwanted effects, you should immediately stop taking POTASSIUM IODIDE RAMKOPHARM 65 mg tablets and immediately seek medical help:
Usually, ADRs by systems when using POTASSIUM IODIDE RAMKOPHARM 65 mg tablets are of unknown frequency, only in relation to skin and skin appendages, the frequency is: RARE – 1 in 1000.
Increased salivation, metallic taste in the mouth, lacrimation, soreness of the teeth and gums, irritation of the stomach lining, headache, swelling of the salivary glands and diarrhea may occur. Serious allergic reactions such as angioedema, arthralgia (joint pain), eosinophilia (change in the white blood count) and swelling of the lymph nodes are observed very rarely and require careful monitoring and discontinuation of the medicinal product. Manifestations of iodism (burning of the mouth and throat, stomach irritation, profuse watery salivation (drooling), severe headache and acne-like rashes and other skin lesions) and potassium toxicity (confusion, stiffness or weakness of the arms and/or legs, fatigue, cardiac arrhythmias) are observed with long-term use of the drug. The risk of suppressing thyroid function in a single dose is insignificant, but when potassium iodide is administered within a few days, reactions of hypothyroidism may occur, especially in newborns and very elderly patients.
Reporting adverse reactions
If you get any side effects, tell your doctor or pharmacist. This includes all possible side effects not described in this leaflet. You can report side effects directly through the national reporting system:
Medicines Executive Agency
8 Damyan Gruev St
1303 Sofia city
Tel. (02) 8903417
E-mail: www.bda.bg.
By reporting side effects, you can contribute to getting more information about the safety of this medicine.
5. HOW TO STORE POTASSIUM IODIDE RAMCOPHARM 65 mg tablets
Keep out of the reach of children.
To be stored at a temperature below 25 o C. To be stored in the original packaging to protect from light and moisture.
l Do not use this medicine after the expiry date which is stated on the blister and carton.
Shelf life - 5 (five) years.
The expiration date corresponds to the last day of the specified month.
Do not dispose of medicines down the drain or in the household waste container. Ask your pharmacist how to dispose of medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACKAGE AND ADDITIONAL INFORMATION
Blister made of hard, colored, opaque PVC and aluminum foil containing 10 tablets.
1 blister in a cardboard box with attached instructions for use.
l What contains POTASSIUM IODIDE RAMKOPHARM 65 mg tablets
- The active substance is potassium iodide. Each tablet contains 65 mg of potassium iodide.
- Excipients are: microcrystalline cellulose, sodium hydrogen carbonate, colloidal anhydrous silica, magnesium stearate.
l What POTASSIUM IODIDE RAMKOPHARM 65 mg tablets looks like and what the package contains
l POTASSIUM IODIDE RAMKOPHARM 65 mg tablets are round, flat, with a dividing line on one side. The tablet can be divided into 2 equal doses.
Primary packaging - 10 tablets each in a PVC/aluminum foil blister.
Secondary packaging – 1 blister in a cardboard box together with a leaflet.
l AUTHORIZATION HOLDER AND MANUFACTURER :
"Ramkofarm" OOD, Plachkovitsa St. 5A, Sofia 1164.
For further information about this medicine, please contact the Marketing Authorization Holder .
Date of last revision of leaflet: MAY 2015 .