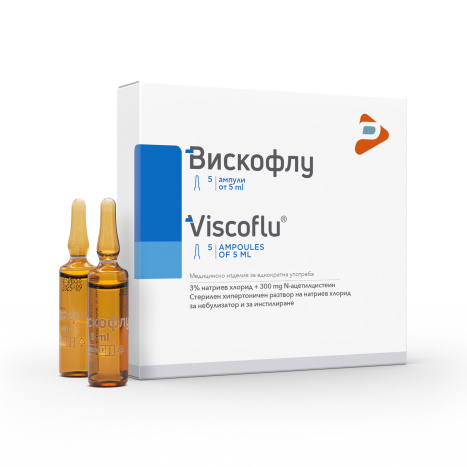
VISCOFLU sterile hypertonic inhalation solution 5ml x 5 amp
3% sodium chloride + 300 mg N-acetylcysteine
Sterile hypertonic solution of
sodium chloride for nebulizer and instillation
AMPOULES OF 5 ml
WITHOUT PRESERVATIVES
1. DESCRIPTION, COMPOSITION AND PURPOSE OF THE MEDICAL DEVICE
1.1 DESCRIPTION AND INDICATIONS
Viscoflu is a single-use medical device intended for use during the treatment of respiratory diseases accompanied by hypersecretion of thick and viscous secretions: acute bronchitis, chronic bronchitis and its complications, pulmonary emphysema, cystic fibrosis, bronchiectasis, sinusitis, secretory inflammation on the ear.
1.2 CONTRAINDICATIONS AND RESTRICTIONS FOR USE
Hypersensitivity to the active substances or to any of the excipients. Viscoflu is contraindicated in children under 2 years of age. Viscoflu should be used during pregnancy or breastfeeding only if absolutely necessary and under direct medical supervision.
1.3 COMPOSITION
One ampoule of 5 ml contains: 300 mg of N-acetylcysteine, 150 mg of sodium chloride. Excipients: sodium hydroxide, disodium edetate, water for injections.
1.4 MECHANISM OF ACTION
Viscoflu works by reducing the density and viscosity of the secretion through the physical/mechanical action of its ingredients:
•sterile pH-controlled hypertonic sodium chloride solution: hypertonic sodium chloride solutions applied directly to the airway mucosa attract water by osmosis from the intracellular to the extracellular space. This increases the percentage of water in the secretion overlying the epithelium, thus thinning it and thus facilitating its excretion.
•N-acetylcysteine: the acetylated derivative of the amino acid cysteine demonstrates a secretolytic action in direct contact with the secretion. This action is due to the presence of sulfhydryl groups in the molecule, which can destroy the disulfide bridges of mucoproteins, which are responsible for the viscosity of mucus, depolymerizing them. N-acetylcysteine also has an antioxidant effect, preserving the tropism of the respiratory epithelium.
2. TYPE OF PACKAGING
• Box with 5 ampoules at 5 ml.
• Box with 10 ampoules at 5 ml.
3. METHOD OF APPLICATION
Viscoflu is a sterile solution that can:
• To inhale nebulized from an aerosol dispenser to reach the bronchi;
•To be administered by direct instillation as ear and nose washes
•To be instilled at the endobronchial level via indwelling catheters, bronchoscopy, etc.
3.1 DOSAGE, METHOD AND PERIOD OF ADMINISTRATION
Application in the form of an aerosol: nebulize one ampoule for one procedure, having to carry out 1-2 procedures per day for 5-10 days. The frequency and dose for one procedure can be varied widely by your doctor by taking
given the clinical and therapeutic effect without the need to significantly differentiate doses in adults or children. Instillation, rinsing of the ears and other cavities: half or one ampoule per application. Endotracheobranchial instillation: 1 ampoule per application, 1-2 times a day or as needed.
3.2 OVERDOSAGE
Too high doses of Viscoflu in the form of inhalations or endotracheobronchial instillation can lead to increased and excessive secretion of fluids, which in patients with suppression of the cough reflex and special sputum can lead to the need for bronchoalveolar lavage.
4. KNOWN ADVERSE REACTIONS
Due to the high concentration of salt in the Viscoflu solution, it can sometimes cause localized irritation that can cause bronchospasm, a burning sensation in the nasal mucosa, rhinorrhea, nausea, vomiting. Among these effects, eliciting the cough reflex within tolerable limits may be desirable, as this stimulates expectoration and clears the airways. Adverse effects associated with Viscoflu usually resolve spontaneously after discontinuation of treatment. Consult your doctor if they persist. Adherence to the instructions in the leaflet reduces the risk. 5. KNOWN INTERACTIONS WITH OTHER MEDICAL PRODUCTS OR DRUGS
In patients treated with drugs containing nitroglycerin, it is appropriate that they consult a doctor before using Viscoflu, because the presence of N-acetylcysteine can cause hypotension with dilation of the temporal arteries and possibly lead to the appearance of headaches; in such a case, blood pressure should be monitored. Viscoflu should not be administered with antitussive drugs, as suppression of the cough reflex may lead to accumulation of bronchial secretions. Viscoflu can be administered together with bronchodilators, vasoconstrictors, etc., but in such a case it should be used for the shortest possible period. It is not recommended to mix antibiotics with Viscoflu solution. The content of N-acetylcysteine in Viscoflu may affect the results of tests to measure the content of salicylates or ketones in the urine. It is recommended to inform your doctor or pharmacist before using Viscoflu if you have recently taken any other medicines, including those available without a prescription.
6. CONDITIONS OF STORAGE
It is recommended to open the Viscoflu ampoule immediately before use. If the solution has been mixed with other drugs, it must be used as soon as possible and cannot be stored. The expiration date marked on the package applies to the unopened package that has been stored properly. Do not use Viscoflu after the expiration date marked on the package. Keep out of the reach of children.
7. WARNINGS
After opening the ampoule, Viscoflu emits a sulfur smell, which does not affect the application of the product in any way. Sometimes the color of the solution can change to pink in the opened ampoule or when it is drawn into the aerosol device, and this does not affect the effect and tolerability of the product. If you have any further questions related to the use of Viscoflu, contact your doctor or pharmacist. Patients with bronchial asthma should be closely monitored during treatment. If bronchospasm occurs, treatment with N-acetylcysteine should be discontinued
immediately and take appropriate treatment.
8. DISPOSAL
When the expiration date has passed or the product has to be discarded, the medical device must be disposed of in accordance with the local waste management requirements of the relevant competent authorities. Do not dispose of the product or its packaging in the environment.
9. LEGEND
Ampoule:
I. = lot number sc. = term of nastiness
NAC = N-acetylcysteine
MD (medical device) = medical device Pharma Line Srl
Via Bertani, 2 – 20154 Milan, Italy
Distributor:
Orbiko Bulgaria EOOA,
Chelopeshko Shose St. 24, 1839 Sofia
Phone: +359 (2) 40 24 500
Email: info.bg@orbico.com
One ampoule of 5 ml contains: 300 mg N-acetylcysteine, 150 mg sodium chloride. Excipients: sodium hydroxide, disodium edetate, water for injections