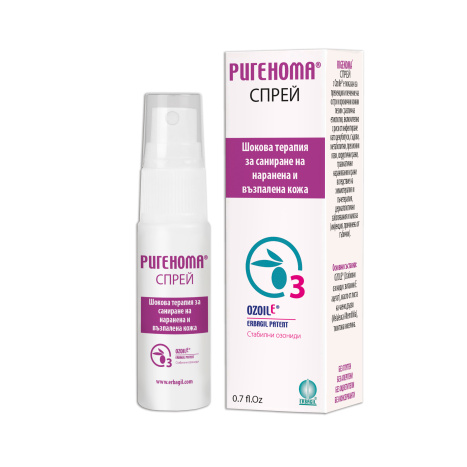
RIGENOMA Spray 20ml
Product: Rigenoma spray 20 ml
Manufacturer: Erbagil srl
Shock therapy for repairing injured and inflamed skin
INDICATIONS
RIGENOMA spray with OZOILE® is indicated for the prevention and treatment of acute and chronic skin lesions of various etiologies, especially those with a risk of infection such as decubitus ulcers, vascular, metabolic, pressure ulcers, surgical wounds, traumatic injuries and wounds resulting from chemotherapy and radiotherapy.
Rigenoma spray is also indicated for the prevention and treatment of dermatological diseases characterized by inflammation, irritation, erythema (redness), xerosis, itching, desquamation (peeling), changes in the flora and barrier function of the skin, possible infection, as in the case of mycosis (infection caused by fungi).
MAIN INGREDIENTS
OZOILE® (stable ozonides with vitamin E acetate), tea tree leaf oil (Melaleuca Alternifolia), thioctic acid.
OZOILE®, stable ozonides with vitamin E acetate, is a pool of innovative molecules, obtained through a patented process, which has anti-inflammatory and antipruritic effects, counteracts hypoxia, creates a hostile environment for the proliferation of pathogenic microorganisms and favors tissue regeneration processes.
HOW TO USE
Spray the product directly onto the affected area.
For the treatment of acute and chronic lesions of various etiologies, after cleaning with Idrozoil detergent, apply Rigenoma spray to the base of the lesion, making one spray every 4 cm², from a distance of approximately 5 cm. To complete the dressing, apply a homogeneous layer of Rigenoma cream to the perilesional skin, forming an area covering more than 5 cm of the surrounding tissue. Cover the wound with sterile gauze smeared with Rigenoma cream to protect it, maintain the environment moist and stimulate tissue repair and regeneration by actively interacting with the wound bed.
Apply Rigenoma spray once or twice daily. Under a bandage (elastic) or occlusive dressing, apply Rigenoma spray when changing the bandage every 3 or 4 days, as directed by your doctor.
When treating dermatological problems, apply Rigenoma spray 2 or 3 times daily.
The treatment period varies depending on the area treated and the speed of the therapeutic response.
WARNINGS AND PRECAUTIONS
Product for topical use. Do not swallow. Keep out of reach of children.
Do not use if the packaging appears damaged. Do not use this medical device after the expiry date. The expiry date refers to an intact (unopened) and properly stored product. Store in a cool, dry place, away from heat sources and direct sunlight. Store preferably at temperatures between 5⁰ and 35⁰C. Wash your hands thoroughly before using the device. Carefully close the vial after use. It is recommended to use the product within 6 months of opening the package.
CONTRAINDICATIONS
Rigenoma spray is well tolerated. It is contraindicated in case of established hypersensitivity to the ingredients. There are no reported cases of sensitization or side effects. If you notice any, please discontinue use and consult your doctor.
There are no known cases of overdose.
There are no known interactions with drugs or medicinal substances.
PACKAGING: Bottle 20 ml - 0.7 fl. OZ.
MEDICAL DEVICE
GLUTEN FREE
ALLERGEN FREE
WITHOUT COLORANTS
WITHOUT PRESERVATIVES
Erbagil srl - Via L. Settembrini, 13
Telese Terme (Bn) - Italy
www.erbagil.com - info@erbagil.com
Distributor: Salvis Pharma Ltd., 5 D.Peshev Str., 1528 Sofia, Bulgaria, tel.: +35929732299
Product: Rigenoma Spray 20 ml
Producer: Erbagil srl
The shock therapy for injured, inflamed skin to sanitize
INDICATIONS
Rigenoma® spray with Ozoile® is indicated for prevention and treatment of acute and chronic skin lesions with different etiology even if there is a risk of infection such as bedsores, vascular, metabolic, pressure ulcers, surgical wounds, traumatic, chemo and radiotherapy injuries.
The product is also indicated in prevention and treatment of dermatological pathologies characterized by inflammation, irritation, erythema, xerosis, itching, desquamation, alterations of flora and skin barrier, possible infection as in the case of mycosis.
MAIN COMPONENTS
OZOILE® (Stable Ozonides with Vitamin E acetate), Melaleuca Alternifolia Leaf Oil, Thioctic Acid.
OZOILE®, stable Ozonides with Vitamin E acetate, is an innovative molecule pool obtained from a patented process with anti-inflammatory and anti-itching action, contrasts hypoxia, creates an unfavorable environment to pathogenic microbial proliferation and promotes tissue repair processes.
HOW TO USE
Spray the product directly on the affected area.
In the treatment of acute and chronic lesions with different etiology, after cleansing with Idrozoil® detergent, apply Rigenoma® spray on the bottom of the lesion, one spray every 4 cm² approximately, at a distance of approximately 5 cm. To complete the dressing, apply a homogeneous layer of Rigenoma® cream on periwound skin and spread a veil on the surrounding region for more 5 cm, finally cover the lesion with sterile gauze covered with Rigenoma® cream to protect, keep the environment humid, promote tissue repair and regeneration actively dialoguing with the wound bottom.
Apply Rigenoma® spray once or twice a day or in case of bandage and occlusive dressing, every 3 or 4 days to change the dressing as indicated by the physician.
In the treatment of dermatological problems, apply Rigenoma® spray 2 or 3 times a day.
The treatment period differs according to the treated area and the rapidity of the therapeutic response.
WARNINGS AND PRECAUTIONS
Topical use. Do not ingest. Keep out of the reach of children. Do not use it if the packaging appears to be damaged. Do not use the device beyond the expiration date. The expiration date refers to the intact and correctly stored product. Store in a cool, dry place, away from heat sources and direct sunlight. Store preferably between 5°C and 35°C. Wash your hands well before using the medical device. Carefully close the tube after use. After opening it is recommended to use the product within 6 months.
CONTRAINDICATIONS
Rigenoma® spray is well tolerated. It is contraindicated in case of ascertained hypersensitivity towards the components. There are no cases of sensitization or side effects, if this phenomenon occurs please discontinue use and consult your physician.
There are no known overdose cases.
There are no known interactions with drugs and medicinal substances.
PACKAGE: Bottle 20 ml - 0.7 fl. OZ.
MEDICAL DEVICE
GLUTEN-FREE
NO COLORS
NO ALLERGENS
NO PRESERVATIVES
Erbagil srl - Via L. Settembrini, 13
Telese Terme (Bn) - Italy
www.erbagil.com - info@erbagil.com